Figure 1

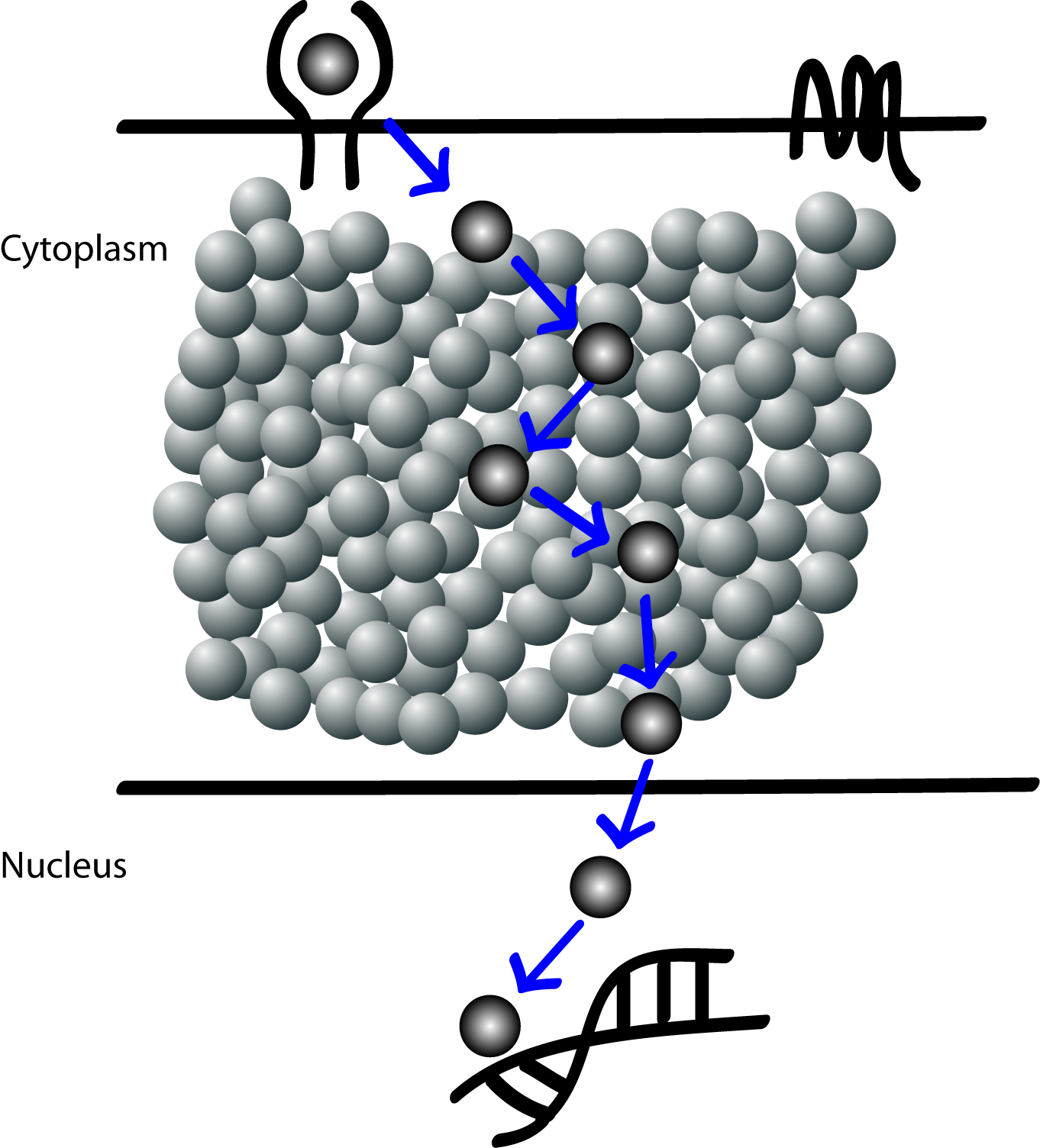
Signal transduction cascades have been traditionally conceived and depicted as linear relay of signals from external environment to the internal environment of a cell resulting in appropriate cell fate decisions.
Figure 4
Critical cell fate decisions are being made at every level of a signal transduction cascade from the receptors to the transcription factors. Such critical proteins in a signaling pathway are usually the ones which 'cross-talk' with other signaling pathways. Many of them are regulated by multisite post-translational mechanisms such as phosphorylation or ubiquitination. In many cases they are regulated my multiple multisite modifications. Hence in order to understand how cells achieve signaling specificity it is important to understand the regulation of these proteins and pathways which are part of many signal transduction pathways. My major research interest is to understand the regulation of these proteins by multisite reversible protein modifications such as phosphorylation, ubquitination, acetylation and so on.
|
|
Figure 2

This notion of insulated linear pathways of signal transduction are no more valid because many of these pathways seem to use components (i.e. proteins) from other pathways and there seem to be little or no insulation at all between components of pathways.
Multisite post translational modifications
Proteins are often thought of as 'on/off' molecular switches in signal transduction cascades and this notion can be mis leading. There are ~150 reversible protein post translational modifications such as phosphorylation, ubiquitination, methylation and so on. Many proteins are modified on multiple sites by the same modification or different modifications. This gives rise to the possiblity of multiple states of proteins. For instance a protein with 2 phosphorylational site can exsist in 4 possible states and hence it is imperative to consider the distribution of these states of proteins in a given cellular context. In the example illustrated, a protein could be modified on two sites by phosphorylation and on a single site by double ubiquitnation. There are 12 possible states in which this protein could exsist and more importantly in many possible distribution of states. My research focuses on this distribution of states of proteins which are multiply modified. More precisely on how does the cell utilise this 'complexity' to encode information. Its our belief that each of this distribution would lead to different cell fate decisions giving rise to 'signaling specifity'.
We use peptide based mass-spectrometry, protein based mass-spectrometry and NMR to unravel the modification states and distributions of proteins. In order to understand the kinetics of these modifications it is important to be able to mathematically analyse and predict such modifications. Hence our approach is a combination of experimental and mathematical analyses. Multisite phosphorylation and signal transduction events would be analysed in in-vitro, cell-culture and model organisms such as hydra, planaria and stentor. Planaria and Hydra undergo re-wiring of their signaling networks during different developmental stages, they also undergo reversible differentiation and regeneration which too indicates re-wiring of networks. Stentor is an unique single cell organism which not only shows amazing regeneration capacity but also exhibits behavioural/learning responses. Behaviour and learning in this 'single-cell' organism could only imply changes in the biochemistry and signal transduction mechanism in the organism since there are no 'synapses' involved for information storage as in the case of specialised neurons in higher organisms. Hence we could term this as a model system for 'cellular cognition'. |
|
Figure 3
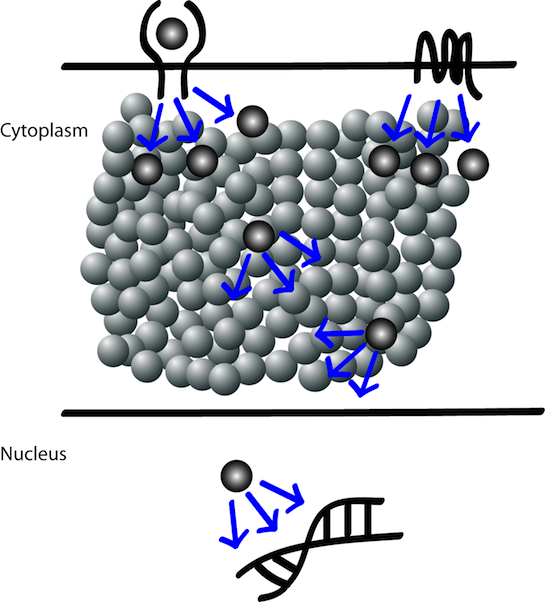
Therefore cartoon diagrams depiciting signal transduction events in a cell could be misleading because the cellular mileau is highly crowded with many proteins (whether being part of the pathway or not) in close proximity with each other. The other most misleading representation in cartoon diagrams is that of a protein shown as 'a single molecule' while they exsist as a population of tens to thousands of molecules. So it is a higly crowded enviornment and it is an enigma how signals follow a desired path from the external environment into the internal cellular mileu without losing its specificity and elliciting the desired cell fate.

|






